Coding guidelines for drug-related medical claims
National drug code information for drug-related medical claims
The current industry standard is to include the National Drug Code (NDC), NDC units, and NDC unit qualifier on all drug-related medical claims. NDC information helps differentiate and identify medications that share the same Healthcare Common Procedure Coding System (HCPCS) code for drug preferences, detect billing errors, and improve reimbursement processes.
In alignment with Centers for Medicare & Medicaid Services (CMS) requirements, we recommend that the following information be included in the designated fields on drug-related medical claims:
- Applicable HCPCS or Current Procedural Terminology (CPT®) codes
- Number of HCPCS or CPT code units
- 11-digit NDC, including the N4 qualifier
- Dosage unit of measurement (F2, GR, ML, or UN)
- Number of NDC units administered/dispensed (must be greater than 0)
NDC coding applies to claims for outpatient services billed with:
- CMS-1500 for providers
- UB-04 for hospitals and facilities
- Electronic data interchange (EDI) 837
Note:This does not affect hospital or facility inpatient claims.
Frequently Asked Questions
- Why are we requesting the NDC, NDC units, and NDC unit qualifiers on provider and outpatient drug-related medical claims?
NDCs are the industry standard identifier for drugs and provide full transparency on the medication administered. They accurately identify the manufacturer, drug name, dosage, strength, package size, and quantity. - Should drugs that are billed through a hospital outpatient department include NDC and NDC units?
Yes. To maintain consistent claim billing guidelines, the NDC would apply to hospital outpatient claims.
- What NDC information should be included on claims?
Claims should include the following information:
- Valid 11-digit NDC number
- NDC unit of measurement (F2, GR, ML, or UN)
- NDC units dispensed/administered (must be greater than 0)
- Where is the NDC located?
The NDC is found on the prescription drug label of the container (e.g., vial, bottle, or tube). The NDC is a universal number that identifies a drug or a related drug item. The NDC number consists of 11 digits with hyphens separating the number into three segments in a 5-4-2 format (e.g., 12345-1234-12). The first five digits identify the manufacturer of the drug and are assigned by the U.S. Food and Drug Administration (FDA). The remaining digits are assigned by the manufacturer and identify the specific product and package size. - What other resources offer information about NDCs?
The FDA package insert for drugs contains information on the NDC. The FDA also has an online searchable National Drug Code Directory and a downloadable NDC Database file at www.fda.gov/drugs/drug-approvals-and-databases/national-drug-code-directory
The Centers for Medicare & Medicaid Services (CMS) has an NDC-to-HCPCS crosswalk (2019 ASP Drug Pricing Files) online at www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2019ASPFiles.html.
- Are NDC units different from HCPCS and CPT code units?
Yes. NDC units are different from HCPCS and CPT code units. You should continue to submit HCPCS codes and service units as they are used to determine reimbursement. NDC units are based on the numeric quantity administered to the patient and the unit of measure (UOM). Refer to the following table for guidance:
UOM* Description General guidelines F2 International Unit International units will be used when billing. GR Gram Grams are usually used when an ointment, cream, inhaler or bulk powder jar are dispensed. It is not typically used for physician-administered drug billing. ML Milliliter If a drug is supplied in a vial in liquid form, bill in millimeters. UN Unit If a drug is supplied in a vial in powder form, bill each vial used.
*Note: ME is also a recognized billing qualifier that may be used to identify milligrams as the NDC unit of measure; however, drug costs are generally created at the UN or ML level. If a drug product is billed using milligrams, it is recommended that the milligrams be billed in an equivalent decimal format of grams.
NDC units
The actual decimal quantity administered and the units of measurement are required on the claim. If reporting a partial unit, use a decimal point. (i.e., if three 0.5 ml vials are dispensed, report ML 1.5).- GR 0.045
- ML 1.5
- UN 2
The number of digits for the quantity is limited to eight digits before the decimal and three digits after the decimal. If entering a whole number, do not use a decimal. Do not use commas or fill in zeros for numbers; leave the remaining fields blank. Please refer to the following examples:- 1234.56
- 2
- 12345678.123
- How do I submit Not Otherwise Classified Drugs (NOCs)?
Example of NOC drugs for box 24G is one of the following:
- Enter the exact same number of HCPCS units as NDC units
- Enter 1 as a default


- What if there are multiple NDCs within an HCPCS code?
If there is more than one NDC utilized within an HCPCS code (i.e., when multiple drug strengths are used), submit each applicable NDC as a separate claim line. Each drug code submitted must have a corresponding NDC, NDC unit, and NDC unit qualifier on each claim line.
If the drug administered is comprised of more than one ingredient (i.e., is a compound or the same drug with different strengths), represent each NDC on a claim line with the appropriate drug code. Standard HCPCS or CPT code billing accepts the use of the following modifiers for determination when more than one NDC is billed for a service code:
Paper claims
- KP – First drug of a multiple drug unit dose formulation
- KQ – Second or subsequent drug of a multiple drug unit dose formulation
- KO – Single drug unit dose formulation
Electronic claims
The compound drug should be reported by repeating the LIN and the CPT segments in the 2410 identification loop. - If the medication comes in a box with multiple vials, should I use the NDC information on the box or the NDC information on the individual vial?
The NDC information requested is from the vial that was administered to the patient along with the appropriate unit of measure and NDC quantity administered.
- How should the NDC, unit of measure, and quantity be submitted?
To submit the NDC, unit of measure, and quantity, follow these directions:
CMS-1500 claim forms (paper)
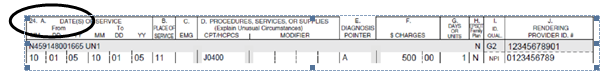
To submit the NDC, unit of measure, and quantity for paper CMS-1500 claim forms, enter the NDC in the shaded area of the service lines in field 24A.
- The six service lines in section 24 have been divided horizontally to accommodate submission of supplemental information to support the billed service. The top portion in each of the six service lines is shaded and is the location for reporting supplemental information.
- The NDC is to be preceded with the qualifier N4 and followed immediately by the 11-digit NDC code (e.g. N412345678901).
When entering supplemental information for NDC, add the following order in this order: N4 qualifier, 11-digit NDC code, one space, 2 character unit/basis of measurement qualifier as applicable (i.e., units 'UN', international units 'F2', gram 'GR' or milliliter 'ML'), and quantity.
- GR 0.045
- ML 1.5
- UN 2
- 1234.56
- 2
- 12345678.123
- Field 42: Revenue code
- Field 43: NDC 11-digit number, Unit of Measurement Qualifier, and Unit Quantity
- Field 44: HCPCS code
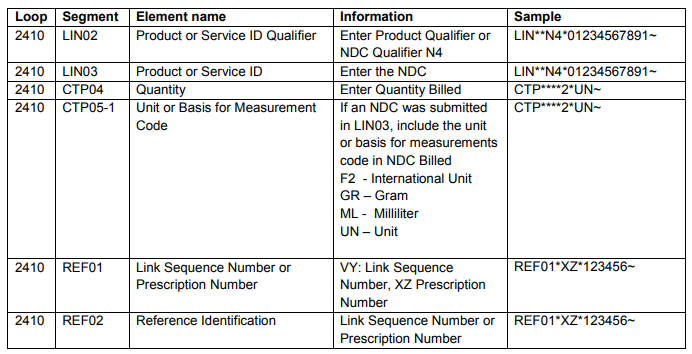
- The NDC qualifier N4 and NDC code are sent in the LIN segment
- LIN02 – NDC Qualifier
- LIN03 – NDC Code
- The quantity and unit of measure are sent in the CTP segment
- CTP04 – Quantity
- CTP05-1 – Unit of Measure
- The prescription number or link sequence number (to report components for a compound drug)
- REF01 - VY: Link Sequence Number, XZ : Prescription Number
- REF02 – Link Sequence Number or Prescription Number
If the NDC on the label does not include a full series of 11 digits, add a leading zero to the appropriate section to create a 5-4-2 configuration. Do not include spaces or hyphens in the NDC digits on the claim. Refer to the following table for examples:
| NDC on label | NDC with addition of leading zero to total 11 digits | Final NDC format to submit on medical claim |
|---|---|---|
| 12345-1234-12 | 12345-1234-12 | 12345123412 |
| 1234-1234-12 | 01234-1234-12 | 01234123412 |
| 12345-123-12 | 12345-0123-12 | 12345012312 |
| 12345-1234-1 | 12345-1234-01 | 12345123401 |
The actual decimal quantity administered and the units of measurement are required on the claim. If you are reporting a partial unit, use a decimal point (i.e., if three 0.5 ml vials are dispensed, report ML 1.5). Please refer to the following for examples:
The number of digits for the quantity is limited to eight digits before the decimal, and three digits after the decimal. If entering a whole number, do not use a decimal. Do not use commas or fill in zeros for numbers; leave the remaining fields blank. Please refer to the following examples:
To submit the NDC, unit of measure, and quantity for paper UB-04 claim forms, please enter the information for each field as follows:

EDI requirements: Professional claims (837 Professional)
When submitting the NDC, unit of measure, and quantity for professional claims, the identification loop is 2410, and:
Please refer to the following table for examples:
Additional information
If you have any questions or need additional information, please call Cigna Customer Service at 1.800.88Cigna (882.4462).